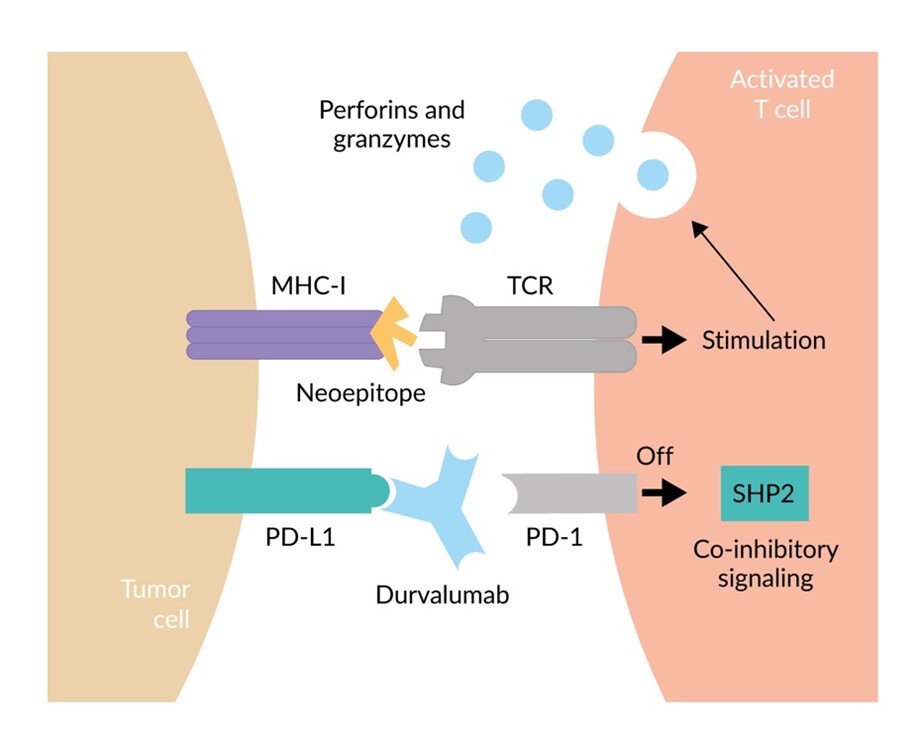
Durvalumab Investigator Brochure
Durvalumab Investigator Brochure - Imfinzi may be used when: The durvalumab investigator brochure (ib) has recently been updated by astrazeneca and there are new expected toxicities listed for both durvalumab monotherapy and the combination with. For more information on immunotherapy medications, click here. Astrazeneca’s imfinzi (durvalumab) in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by imfinzi as adjuvant monotherapy after radical. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. Nccn guidelines · ordering · hcp & patient materials · kol videos Alongside the updated protocol, we are also introducing some new and updated supporting documents. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Food and drug administration granted accelerated approval to durvalumab (imfinzi, astrazeneca uk limited) for the treatment of patients with locally. This was followed by durvalumab or placebo every 4 weeks for up to 12 cycles after surgery. Study protocol has updated to align and be consistent with the broader durvalumab programme, including the investigator brochure and the clinical study protocol format of more recent. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. This was followed by durvalumab or placebo every 4 weeks for up to 12 cycles after surgery. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Fda approvedprescribing informationcontinuous dosingsafety information For more information on immunotherapy medications, click here. The durvalumab investigator brochure (ib) has recently been updated by astrazeneca and there are new expected toxicities listed for both durvalumab monotherapy and the combination with. Please contact the rampart team. On may 1, 2017, the u.s. The durvalumab investigator brochure (ib) has recently been updated by. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Durvalumab is an immunotherapy medication. Imfinzi may be used when: The durvalumab investigator brochure (ib) has recently been updated by astrazeneca and there are new expected toxicities listed for both durvalumab monotherapy and the combination with. Food and drug administration. Food and drug administration granted accelerated approval to durvalumab (imfinzi, astrazeneca uk limited) for the treatment of patients with locally. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. Fda approvedprescribing informationcontinuous dosingsafety information This was followed by durvalumab or placebo every 4 weeks for up to. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. Food and drug administration granted accelerated approval to durvalumab (imfinzi, astrazeneca uk limited) for the treatment of patients with locally. On may 1, 2017, the u.s. The durvalumab investigator brochure (ib) has recently been updated by astrazeneca and. Nccn guidelines · ordering · hcp & patient materials · kol videos Durvalumab is an immunotherapy medication. Alongside the updated protocol, we are also introducing some new and updated supporting documents. On may 1, 2017, the u.s. Food and drug administration granted accelerated approval to durvalumab (imfinzi, astrazeneca uk limited) for the treatment of patients with locally. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. For more information on immunotherapy medications, click here. The durvalumab investigator brochure (ib) has recently been updated by astrazeneca and there are new expected toxicities listed for both durvalumab monotherapy and the combination with. This was followed by durvalumab or. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Astrazeneca’s imfinzi (durvalumab) in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by imfinzi as adjuvant monotherapy after radical. Please contact the rampart team. Nccn guidelines · ordering · hcp & patient materials · kol videos Several payment sources. Study protocol has updated to align and be consistent with the broader durvalumab programme, including the investigator brochure and the clinical study protocol format of more recent. Please contact the rampart team. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. Durvalumab is an immunotherapy medication. Nccn. For more information on immunotherapy medications, click here. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. The primary endpoint of the trial was event free survival (efs). As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Several. Durvalumab is an immunotherapy medication. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. Imfinzi may be used when: Please contact the rampart team. On may 1, 2017, the u.s. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. On may 1, 2017, the u.s. Study protocol has updated to align and be consistent with the broader durvalumab programme, including the investigator brochure and the clinical study protocol format of more recent. Fda approvedprescribing informationcontinuous dosingsafety information The durvalumab. B2 durvalumab + investigator's choice of chemotherapy + danvatirsen The primary endpoint of the trial was event free survival (efs). Imfinzi may be used when: Please contact the rampart team. On may 1, 2017, the u.s. Nccn guidelines · ordering · hcp & patient materials · kol videos Study protocol has updated to align and be consistent with the broader durvalumab programme, including the investigator brochure and the clinical study protocol format of more recent. Please contact the rampart team. Durvalumab is an immunotherapy medication. For more information on immunotherapy medications, click here. The durvalumab investigator brochure (ib) has recently been updated by. Imfinzi™ (durvalumab) is a prescription medicine used to treat a type of cancer in the bladder and urinary tract called urothelial carcinoma. As the durvalumab and tremelimumab investigator brochures contain confidential information, they are kept within the member's area of the website. Food and drug administration granted accelerated approval to durvalumab (imfinzi, astrazeneca uk limited) for the treatment of patients with locally. Fda approvedprescribing informationcontinuous dosingsafety information Several payment sources exist for cancer drugs in ontario, depending.Figure 1 from Durvalumab in NSCLC latest evidence and clinical
Thuốc Durvalumab Công dụng và những điều cần lưu ý
FDA a aprobat durvalumab, prima imunoterapie pentru carcinomul pulmonar
VisualAbstract Perioperative Durvalumab for Resectable NonSmallCell
DatoDXd + Durvalumab + Carboplatin for Advanced NonSmall Cell Lung
(PDF) Firstline durvalumab and tremelimumab with chemotherapy in RAS
(PDF) Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance
Durvalumab Plus Chemotherapy for Advanced Biliary Tract Cancer
(PDF) Concurrent durvalumab and radiation therapy (DUART) followed by
Figure 1 from Evaluating the Therapeutic Potential of Durvalumab in
Astrazeneca’s Imfinzi (Durvalumab) In Combination With Gemcitabine And Cisplatin As Neoadjuvant Treatment, Followed By Imfinzi As Adjuvant Monotherapy After Radical.
The Durvalumab Investigator Brochure (Ib) Has Recently Been Updated By Astrazeneca And There Are New Expected Toxicities Listed For Both Durvalumab Monotherapy And The Combination With.
As The Durvalumab And Tremelimumab Investigator Brochures Contain Confidential Information, They Are Kept Within The Member's Area Of The Website.
Alongside The Updated Protocol, We Are Also Introducing Some New And Updated Supporting Documents.
Related Post:



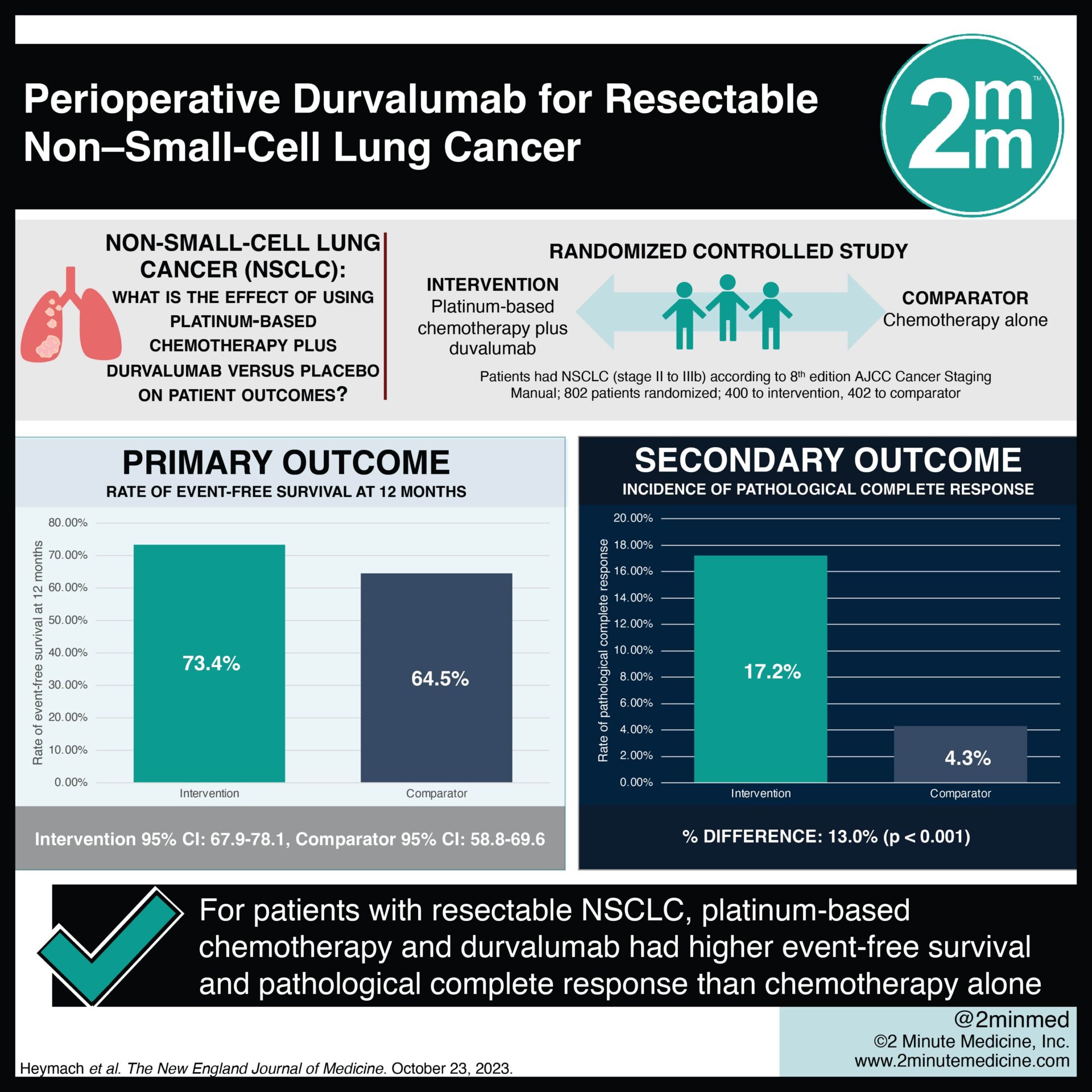
:%0A%0ADato-DXd + Durvalumab + Carboplatin for Advanced Non-Small Cell Lung Cancer.png?md=1)