Pipeline Vantage Brochure
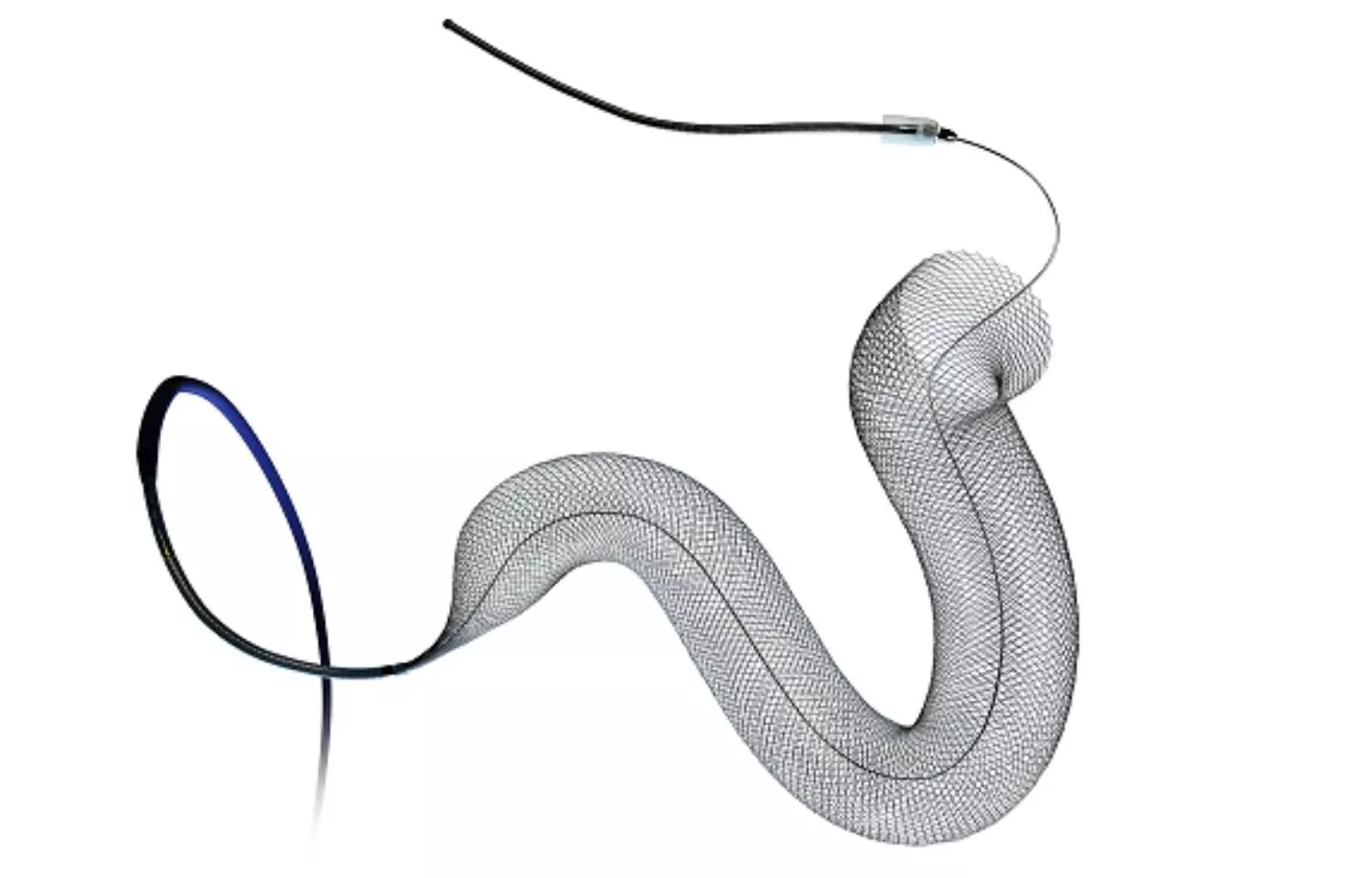
Pipeline Vantage Brochure - Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. Vantage neb pipeline vantage system canada operating pembina pipeline! The newer pipeline generations have. We report here the in vivo biocompatibility and in vitro hemocompatibility performance of the pipeline vantage embolization device with shield technology (vantage) compared with the. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of north dakota,. Vantage is a subsidiary of pembina pipeline corporation. Proved effectiveness up to 6 hours and subsequently to 16 hours with. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. A new study, published in the journal of neurointerventional surgery (jnis) late last year, has assessed the safety of the latest iteration of the pipeline vantage embolisation. Vantage is a subsidiary of pembina pipeline corporation. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. Proved effectiveness up to 6 hours and subsequently to 16 hours with. Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. Endovascular techniques for the minimally invasive treatment of aneurysms and strokes have evolved rapidly. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of north dakota,. Pipeline™ is the reference in flow diversion, with more than 10 years of. The newer pipeline generations have. We report here the in vivo biocompatibility and in vitro hemocompatibility performance of the pipeline vantage embolization device with shield technology (vantage) compared with the. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. The pipeline vantage embolization device (pved) is a novel coated flow diverter with reduced wire diameters to improve neoendothelialization and stent porosity. Pipeline™ is the reference in flow diversion, with more than 10 years of. The pipeline vantage embolization device with shield technology. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. The newer pipeline generations have. We report here the in vivo biocompatibility and in vitro hemocompatibility performance of the pipeline vantage embolization device with shield technology (vantage) compared with the. Vantage is a subsidiary of. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. Pipeline™ is the reference in flow diversion, with more than 10 years of. The newer pipeline generations have. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of north dakota,. A new study, published in the journal of neurointerventional surgery (jnis) late last year, has assessed the safety of the latest iteration of the pipeline vantage embolisation. Proved effectiveness up to 6 hours. Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. The pipeline embolization device is a safe and effective treatment option for intracranial aneurysms. We report here the in vivo biocompatibility and in vitro hemocompatibility performance of the pipeline vantage embolization device with shield technology (vantage) compared with the. Vantage neb pipeline vantage system canada operating pembina pipeline! Pipeline™. The newer pipeline generations have. A new study, published in the journal of neurointerventional surgery (jnis) late last year, has assessed the safety of the latest iteration of the pipeline vantage embolisation. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. Is comprised of. Proved effectiveness up to 6 hours and subsequently to 16 hours with. Vantage is a subsidiary of pembina pipeline corporation. The pipeline vantage embolization device (pved) is a novel coated flow diverter with reduced wire diameters to improve neoendothelialization and stent porosity. We report here the in vivo biocompatibility and in vitro hemocompatibility performance of the pipeline vantage embolization device. Proved effectiveness up to 6 hours and subsequently to 16 hours with. Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. The pipeline embolization device is a safe and effective treatment option for intracranial aneurysms. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. We report here the in vivo. Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. Pipeline™ is the reference in flow diversion, with more than 10 years of. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. The newer pipeline generations have. We report here the in vivo. The pipeline vantage embolization device (pved) is a novel coated flow diverter with reduced wire diameters to improve neoendothelialization and stent porosity. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of north dakota,. The pipeline embolization device is a safe and effective treatment option. The pipeline vantage embolization device with shield technology is a next generation flow diverter developed to improve aneurysm occlusion and implant endothelialization in addition to. Medtronic pipeline™ vantage embolization device with shield technology™ for intracranial aneurysms. Pipeline™ is the reference in flow diversion, with more than 10 years of. Is comprised of approximately 128 miles of pipeline, originating from gas plants in tioga and stateline, north dakota and extending to the northwest corner of north dakota,. The pipeline vantage embolization device (pved) is a novel coated flow diverter with reduced wire diameters to improve neoendothelialization and stent porosity. Proved effectiveness up to 6 hours and subsequently to 16 hours with. The newer pipeline generations have. Endovascular techniques for the minimally invasive treatment of aneurysms and strokes have evolved rapidly. Vantage pipeline us lp (vantage) is the operator of the vantage pipeline and the west spur lateral. Vantage neb pipeline vantage system canada operating pembina pipeline! Vantage is a subsidiary of pembina pipeline corporation.Master & Fellow Pipeline™ Vantage with Shield Technology™ for
Master & Fellow Pipeline™ Vantage with Shield Technology™ for
Preclinical safety and efficacy evaluation of the Pipeline Vantage
Discover Unbeatable Functionality with Professional Sales Bag A
First clinical multicenter experience with the new Pipeline Vantage
Medtronic India unveils Pipeline Vantage Embolization Device with
(PDF) Preclinical safety and efficacy evaluation of the Pipeline
First clinical multicenter experience with the new Pipeline Vantage
Pipeline™ Vantage Embolization Device with Shield Technology™ for Brain
Master & Fellow Pipeline™ Vantage with Shield Technology™ for
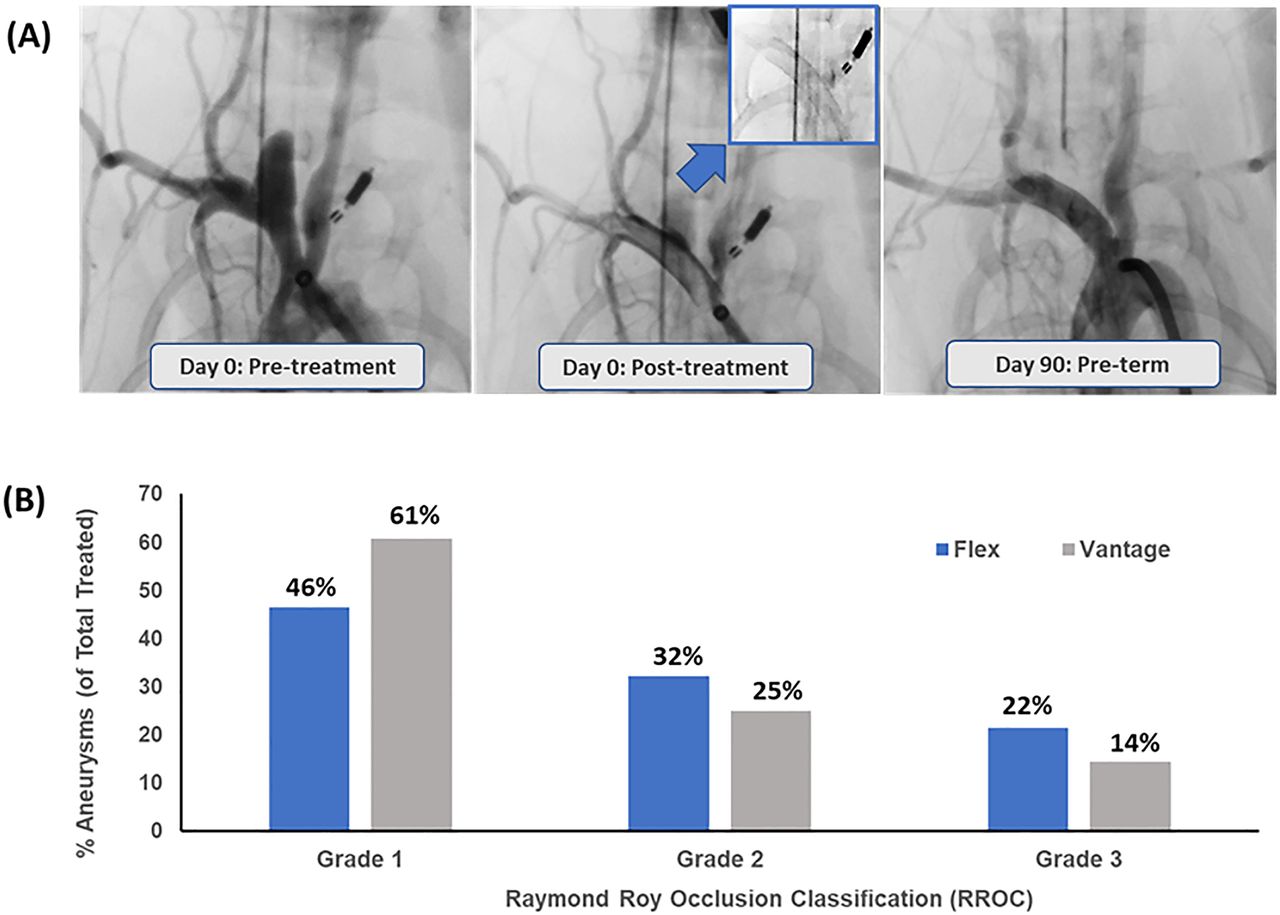
A New Study, Published In The Journal Of Neurointerventional Surgery (Jnis) Late Last Year, Has Assessed The Safety Of The Latest Iteration Of The Pipeline Vantage Embolisation.
The Pipeline Embolization Device Is A Safe And Effective Treatment Option For Intracranial Aneurysms.
We Report Here The In Vivo Biocompatibility And In Vitro Hemocompatibility Performance Of The Pipeline Vantage Embolization Device With Shield Technology (Vantage) Compared With The.
Related Post: